Helonium: The Universe’s First and Fiercest Molecule
What if I told you the very first molecule in the universe was also the strongest acid ever known? A substance so reactive it defies containment, a chemical ghost that haunted scientists for a century. This isn’t science fiction. It’s the incredible true story of helonium.
This article demystifies the helium hydride ion (HeH+), a molecule more commonly known by its striking alias, helonium. We will journey beyond dense scientific jargon to uncover its profound importance. You will learn why it earned the title of the universe’s primordial molecule, what makes it the undisputed king of acids, and how scientists finally captured this cosmic phantom after a 100-year hunt.
Get ready to travel back to the dawn of time. We will witness the birth of chemistry itself and grasp the deep implications of this tiny, yet monumentally powerful, molecule. The story of helonium is the story of how our complex cosmos began.
The Dawn of Everything: Why Helonium Was the Universe’s First Molecule
The young universe was a chaotic place. For about 380,000 years after the Big Bang, it was an unimaginably hot, dense soup of fundamental particles. Bare protons (hydrogen nuclei), helium nuclei, and a sea of electrons zipped around, too energetic to settle down and form the atoms we know today.
But as the universe expanded, it cooled. This cooling changed everything. Helium nuclei, holding a stronger positive charge than hydrogen, had a greater pull on the free-floating electrons. They were the first to capture an electron, forming the first neutral atoms. This happened when the cosmos cooled to about 4000 Kelvin.
It was in this critical period that the first molecular bond in history was forged. A neutral helium atom, stable but drifting, encountered a bare, lonely proton. They combined. The result was HeH+, the helium hydride ion, or helonium. It was the universe’s first, tentative step toward chemical complexity.
This primordial molecule had a crucial, if fleeting, role. Helonium readily reacted with the newly forming neutral hydrogen atoms. In this reaction, it donated its proton, forming molecular hydrogen (H2) and leaving a neutral helium atom behind. This H2 was the essential fuel that would later collapse under gravity to ignite the very first stars, ending the cosmic dark ages.
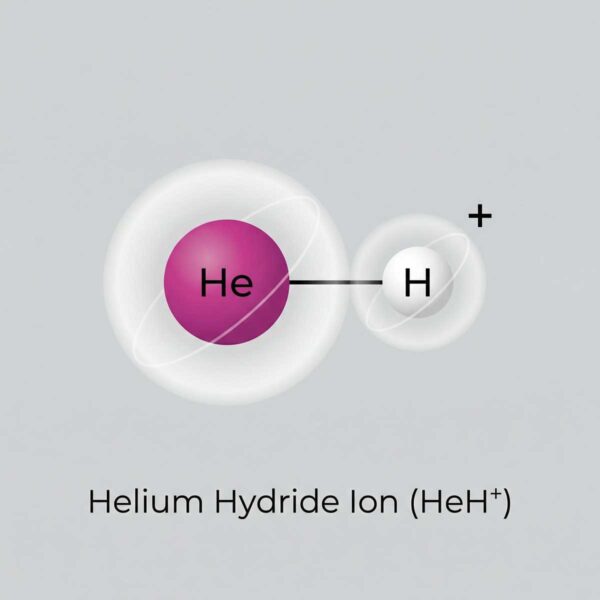
The Undisputed Champion: What Makes Helonium the Strongest Acid?
To understand helonium’s power, we first need a simple definition of acidity. Think of it as a chemical’s overwhelming desire to give away a proton. A weak acid might reluctantly let one go. A strong acid is eager. A superacid, like helonium, is a proton-donating force of nature, like a person desperate to get rid of a hot potato.
Helium is a noble gas. It is chemically aloof, perfectly content with its full shell of electrons. It hates sharing. When forced into a bond with a proton to form helonium, the helium atom is in an extremely uncomfortable state. It will do anything to get rid of that extra proton and return to its preferred, stable, neutral state.
This desperation is measured by a value called pKa. The lower the pKa, the stronger the acid. While 100% sulfuric acid has a pKa of -3, helonium’s is a staggering -63. This isn’t just a small difference; it’s an almost unimaginable leap in chemical reactivity. To put that number in perspective, let’s compare it to other famous superacids.
Helonium vs. Other Superacids
| Acid | Chemical Formula | pKa (approx.) | Strength Comparison |
|---|---|---|---|
| Helonium (Helium Hydride Ion) | HeH+ | -63 | The strongest known acid in existence. |
| Fluoroantimonic Acid | HSbF6 | -28 | Billions of times weaker than helonium. |
| Magic Acid | FSO3H-SbF5 | -23 | Extremely strong, but still far weaker than helonium. |
| 100% Sulfuric Acid | H2SO4 | -3 | A common strong acid, but incomparable to helonium. |
| Stomach Acid (Hydrochloric Acid) | HCl | 1.5 | Weak by comparison, yet strong enough to digest food. |
This extreme reactivity leads to a simple, mind-boggling rule: helonium cannot be contained. It will protonate, and thus decompose, any substance it touches. This makes it a fascinating entity, one that can only be studied in the vacuum of space or created for fleeting moments in highly controlled laboratory settings.
The 100-Year Hunt: How Scientists Finally Found a Cosmic Ghost
The story of helonium begins not in the cosmos, but in a laboratory. In 1925, chemists T. R. Hogness and E. G. Lunn at the University of California, Berkeley, first created the HeH+ ion. They established its existence, but it remained a chemical curiosity, a theoretical possibility.
Astronomers in the 1970s began to suspect this molecule should exist in interstellar space. Their models of cosmic chemistry predicted it. Yet, for decades, every search was fruitless. This created a troubling dilemma: if this fundamental molecule couldn’t be found, was our understanding of the chemistry of the universe flawed?
The breakthrough came from above. The Stratospheric Observatory for Infrared Astronomy, or SOFIA, provided the key. SOFIA is a Boeing 747SP aircraft modified to carry a large telescope. By flying at altitudes up to 45,000 feet, it soars above the water vapor in Earth’s atmosphere that blocks most infrared light from reaching the ground.
In April 2019, a team of scientists pointed SOFIA towards a planetary nebula known as NGC 7027. This nebula, the remnant of a dying sun-like star, provided the perfect natural laboratory. Its intense radiation and specific chemical composition created an environment where helonium could form and, crucially, be observed. After a century of searching, the ghost was found. The specific infrared signature of helonium was detected, confirming its existence in the cosmos.
Beyond the Big Bang: Why Does Helonium Still Matter?
Finding a single type of molecule in a distant nebula might seem like a minor academic victory. But the confirmation of helonium’s existence has profound implications. It serves as a cosmic yardstick, validating our fundamental models of early universe chemistry. If our theories about the very first molecule are correct, it gives us immense confidence in our larger story of how the first stars and galaxies came to be.
Furthermore, studying such an extreme molecule pushes the boundaries of our chemical knowledge. Helonium forces chemists to explore the absolute limits of chemical bonds and reactivity. It provides a benchmark against which all other acids are measured. This theoretical exploration helps refine our understanding of how atoms interact under the most intense conditions imaginable.
Recent research has also revealed that the reaction rates of helonium with hydrogen were faster than previously predicted, which has important implications for understanding the timeline of early star formation. Each new discovery about this primordial molecule adds another piece to the puzzle of our cosmic origins.
FAQ: Your Helonium Questions, Answered
A Legacy of Firsts
Helonium is far more than a chemical curiosity. It is the universe’s first molecular bond, the strongest acid imaginable, and a powerful testament to the patient, persistent journey of scientific discovery. From a fleeting particle in the post-Big Bang inferno to a confirmed cosmic signal a hundred years in the making, its story is our story—the story of how complexity arose from simplicity.
This single molecule connects the physics of the Big Bang to the chemistry of the stars. Understanding helonium helps us understand ourselves, for we are made of stardust forged in furnaces that were first ignited by the chemistry that helonium made possible. Intrigued by the chemistry of the cosmos? Explore the wonders of planetary nebulae on NASA’s Hubblesite or dive deeper into the world of extreme chemistry with resources from the American Chemical Society.
References
- Güsten, R., et al. (2019). “Astrophysical detection of the helium hydride ion HeH+.” Nature, 568, 357-359. https://www.nature.com/articles/s41586-019-1090-x
- Fortenberry, R. C. (2020). “The First Molecule in the Universe.” Scientific American, 322(2), 42-47. https://www.scientificamerican.com/article/the-first-molecule-in-the-universe/
- American Chemical Society. (2019). “Helium hydride – Molecule of the Week.” https://www.acs.org/molecule-of-the-week/archive/h/helium-hydride.html
- Hogness, T. R., & Lunn, E. G. (1925). “The ionization of hydrogen by electron impact.” Physical Review, 26(1), 44-55.
- Corless, V. (2025). “Early universe chemistry: helium hydride reactions faster than expected.” Chemistry World. https://www.chemistryworld.com/news/chemical-reaction-almost-as-old-as-the-universe-was-faster-than-thought/4022191.article
- Wikipedia Contributors. (2024). “Helium hydride ion.” In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Helium_hydride_ion


